5+ orbital diagram for neon
He 2s 2 2p 6. The Basics of Orbital Diagrams.

Neon Orbital Diagram Electron Configuration And Valence Electrons
In the case of Neon we have the electron configuration is He 2s2 2p6 for this particular element.

. Neon is the tenth element with a total of 10 electrons. 1s 2 2s 2 2p 6. So the electron configuration of.
What is incorrect about this orbital diagram. Both arrows in the 2p box should be pointing up. These orbitals are filled with electrons the amount of electrons depends on which.
The atomic number of an element is the number of electrons and protons in that element. There are different types of orbitals that all have different energy levels. The shorthand electron configuration for Neon is He 2s 2 2p 6.
Ground state electron configuration of iodine is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5. The ground-state electron configuration of the Neon Ne atom is 1s22s22p6. The full orbital diagram for neon is shown.
Reduced electronic configuration Ne. Orbital diagrams Orbital box diagrams of all elements are mentioned in the chart given below. Orbital diagrams Orbital box diagrams of all elements are mentioned in the.
The orbital diagram will be filled in the same order as described by the Aufbau principle. In the 2p box there should only be 1 electron in the first 2p box and one in the 2nd 2p box. This is the final precise form for the electron configuration of the element.
What is the orbital diagram for iodine. Heres how you can draw the orbital diagram of neon step by step. Both arrows in the 2p box should be pointing up.
Find electrons of neon. Chemistry questions and answers. Write electron configuration of neon.
Electronic configuration of the Neon atom. The number of electrons in the atom is. There is nothing incorrect with this diagram.
In the iodine ground-state electron configuration five. 000084 gcm 3. The electron configuration of neon is.
In writing the electron configuration for neon the first two electrons will go in. In the 2p box there. Fill in the orbital energy diagram for the neon atom.
1s 2s. What is an orbital diagram. And for the excited state it is 1s 2 2s 2 2p 5 3s 1.
The electron configuration of an atom is 1s 2 2s 2 2p 6. All the arrows should be pointing up. The order in which the orbitals are filled with electrons from lower energy to higher energy is.
That is the number of electrons and protons in the sodium atom is eleven. Below is the electronic diagram. What is incorrect about this orbital diagram.
There is nothing incorrect with this diagram. An orbital diagram is similar to electron configuration except that instead of indicating the atoms. 2p El 2s 1s Submit Answer Try Another Version 2 item attempts remaining.
The above orbital diagram shows that the 1s subshell has 2 electrons the 2s subshell has 2 electrons and the 2p subshell has 6 electrons.
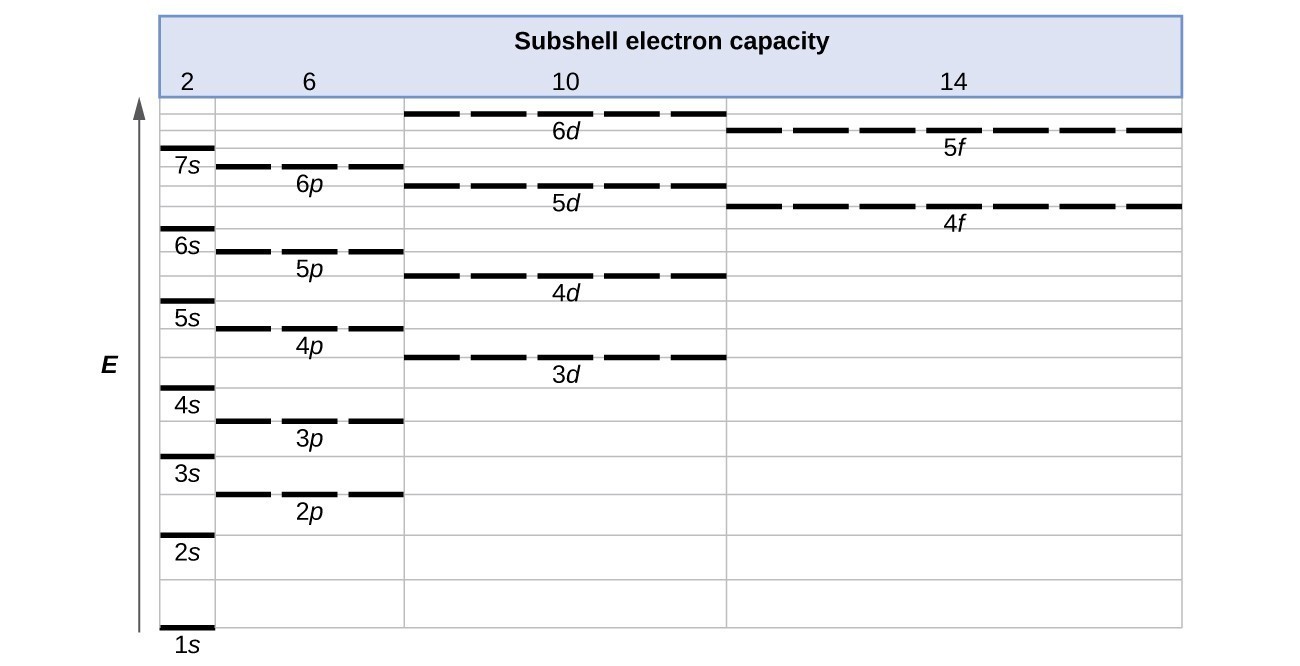
Electronic Structure Of Atoms Electron Configurations General Chemistry I Course Hero
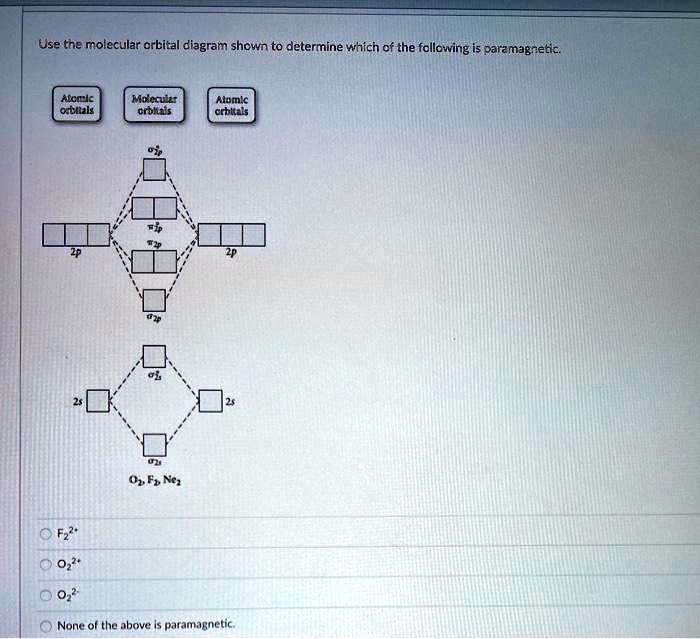
Solved Use The Molecular Orbital Diagram Shown To Determine Which F The Follcwing Is Parzmagretic Accclc Obllake Mdeal Crukeb Alomkc Crbla Ofi N None O The Above Paramagnetic

How To Write The Orbital Diagram For Neon Ne Youtube

3 1 Electron Configurations Chemistry Libretexts

Building Up The Periodic Table

Create An Orbital Diagram For Nitrogen Neon Ppt Download
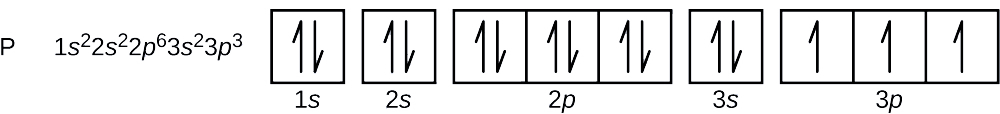
8 3 Electron Configurations How Electrons Occupy Orbitals Chemistry Libretexts

Neon Orbital Diagram Electron Configuration And Valence Electrons
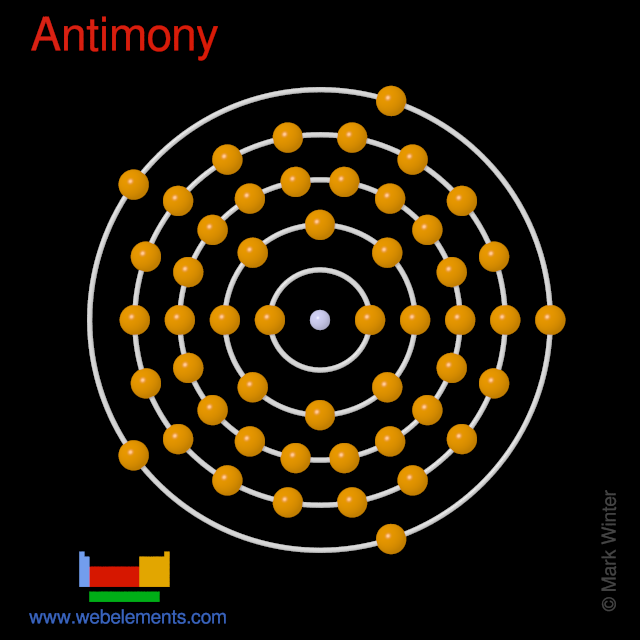
Webelements Periodic Table Antimony Properties Of Free Atoms

Noble Gas Configuration Video Khan Academy
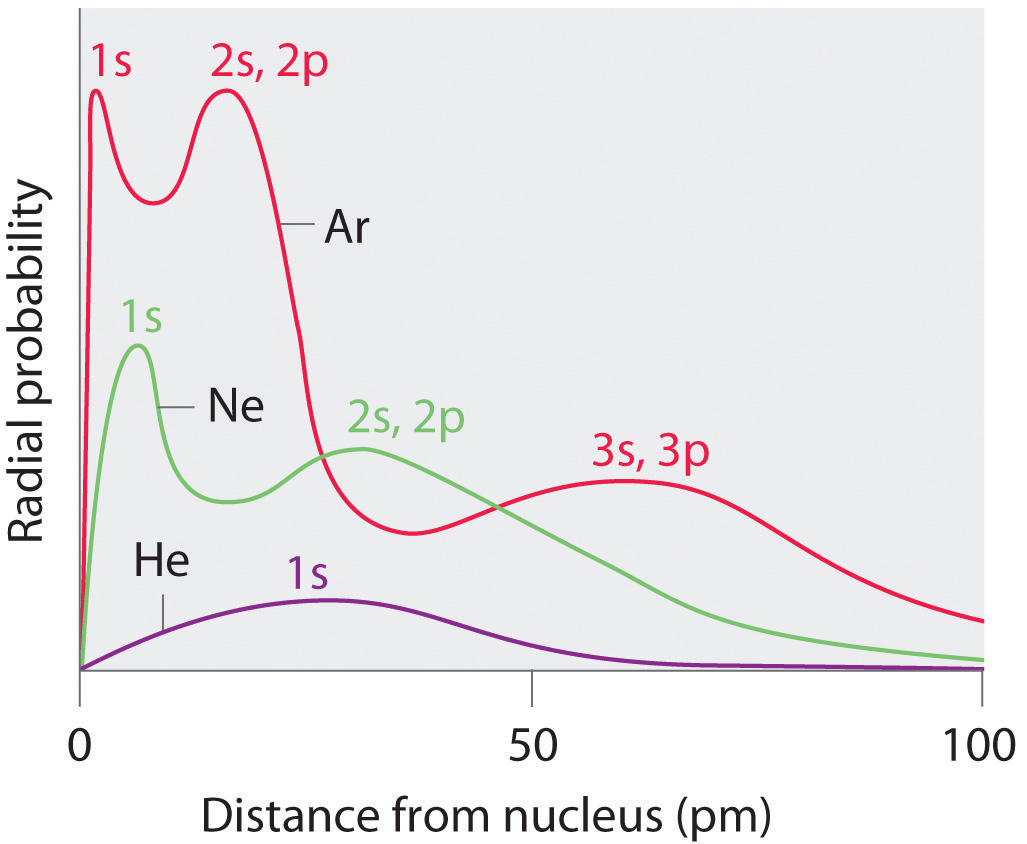
Sizes Of Atoms And Ions

Neon Orbital Diagram Electron Configuration And Valence Electrons

List Of Electron Configurations Of Elements
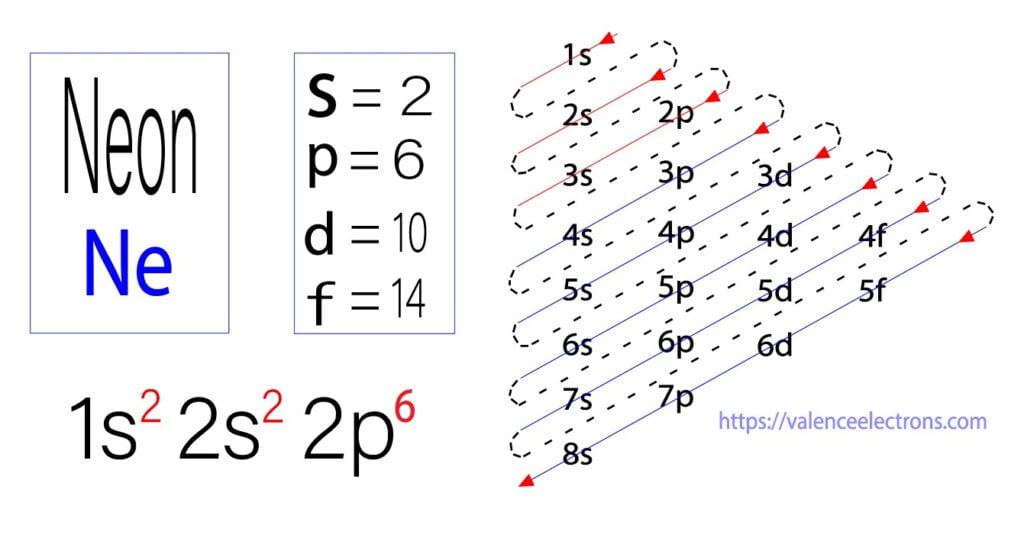
Neon Ne Electron Configuration And Orbital Diagram

Phosphorus Orbital Diagram Electron Configuration And Valence Electrons
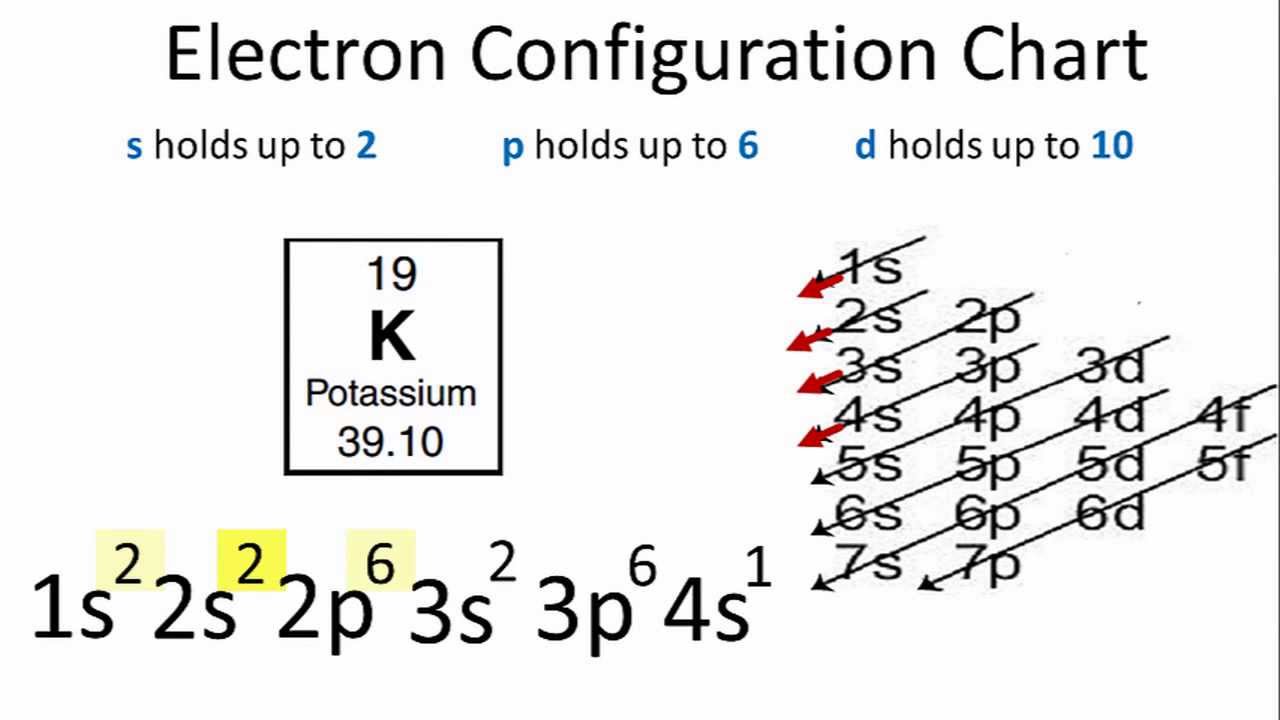
Electron Configuration For Potassium K

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes